The high mortality rate of lung cancer can be attributed to its tendency to metastasize at an early stage, spreading from the lungs to distant organs.
Transforming growth factor β1 (TGFβ1) plays both tumor-suppressing and tumor-promoting roles in cancer biology, and overexpression of TGFβ1 promotes tumor growth and aggressive lung metastasis at late stages of lung cancer development. After surgical removal of NSCLC, the level of TGFβ1 expression serves as a crucial prognostic indicator. However, there has been a significant amount of interest in the recent discoveries of fucoidan’s anti-inflammatory, anti-proliferative, and anti-cancer/anti-tumor properties.
So, in this blog, I would like to share the following study “Fucoidan inhibition of lung cancer in vivo and in vitro: role of the Smurf2-dependent ubiquitin-proteasome pathway in TGFβ receptor degradation” by Hsien-Yeh Hsu et al. According to the study, fucoidan has been shown to decrease tumor size in male C57BL/6 mice with LLC1-xenograft.
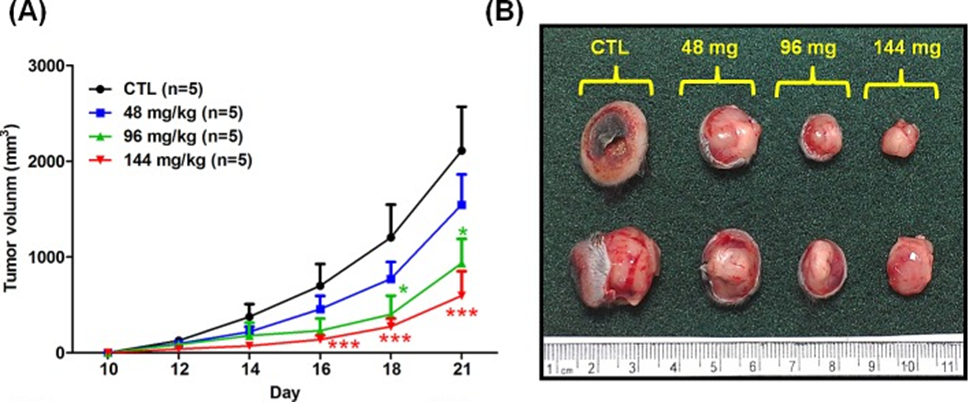
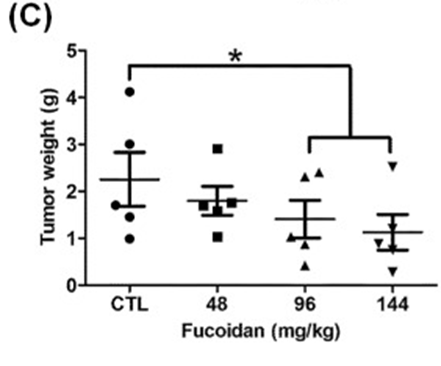
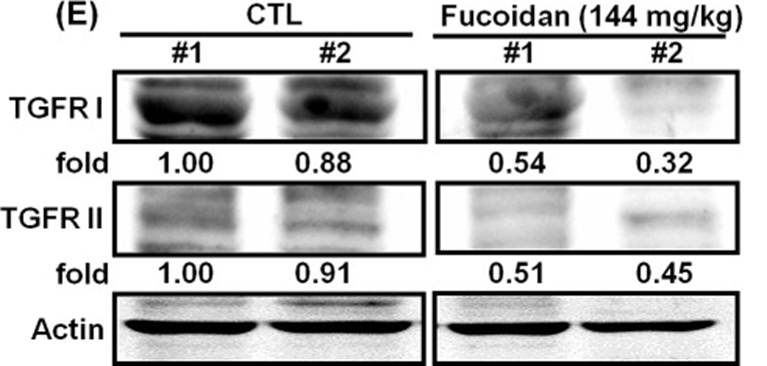
The first step involved studying the effects of orally administered fucoidan on xenografted mice with Lewis lung carcinoma (LLC1) in vivo. Male C57BL6 mice were given subcutaneous injections of LLC1 cells on their dorsum, and the researchers observed the rate of tumor growth for 21 days. The researchers discovered that mice who were given fucoidan experienced a notable decrease in tumor volume (as shown in Figures 1A and 1B) and tumor weight (as depicted in Figure 1C), with the reduction being dependent on the dosage. The researchers used Western blot analysis to confirm TGFR expression in tumor lesions. Our results showed that mice with LLC1 tumors who received fucoidan had lower expression of TGFR (TGFRI and II) compared to the control group, as shown in Figure 1E. This indicates that fucoidan down-regulates TGFRI and TGFRII protein expression in tumor lesions in vivo. The combined results of this study indicate that fucoidan effectively suppresses tumor development and decreases the expression of TGFR protein in a mouse model with LLC1 tumors.
Tumor volumes in mice that orally ingested fucoidan during tumor-bearing mouse growth were compared with those in mice that did not. Tumor volumes in mice that orally ingested fucoidan or ddH2O (CTL) throughout the study period were compared among three treatment groups: continuous feeding (EXP 3), discontinuous feeding (EXP 2), and feeding starting on day 13 (EXP 1). The findings demonstrated that fucoidan had the ability to reduce tumor growth in mice with LLC1 tumors in the EXP 1 group, in contrast to the CTL group. This suggests that administering fucoidan orally on a regular basis can effectively inhibit the formation of lung tumors in mice.
They next investigated the effects of fucoidan on human NSCLC cells, A549 and CL1-5, and mouse LLC1 cells in vitro. By utilizing MTT assays, we conducted a thorough investigation into the impact of fucoidan on the proliferation patterns of lung cancer cells. The results revealed that after 24, 48, and 72 hours of treatment with fucoidan (100 and 200 μg/ml), the cell viability in these cells was significantly reduced by 40-60% in comparison to the control group, as shown in Figure 2A.
They investigated whether fucoidan suppressed the activity of Smad and non-Smad pathways, and as shown in Figure 2B, fucoidan reduced the phosphorylation of Smad2/3, Akt, and Erk in CL1-5 cells. Subsequently, by employing Akt and Erk inhibitors, they discovered that inhibiting Akt and Erk signaling completely eliminated the enhanced cell viability induced by TGFβ1. We used TGFβ1 to stimulate the TGFR downstream signaling pathway and found that TGFβ1 stimulation enhanced phosphorylation of Smad2/3, Akt, and Erk in NSCLC. However, fucoidan still inhibited TGFβ1-induced phosphorylation of these molecules, as shown in Figure 2C. The data, when analyzed together, suggest that fucoidan has the ability to inhibit cell viability in lung cancer cells. This inhibition is achieved by targeting TGFR and subsequently attenuating the Smad2/3, Akt, and Erk signaling pathways.
To explore how fucoidan reduces TGFR in tumor lesion samples isolated from LLC-bearing mice, they examined the effect of fucoidan on TGFRI/II protein expression in various lung cancer cells, including CL1-5, A549, and LLC1. Western blotting analysis showed that fucoidan rapidly reduced TGFRI and TGFRII protein expression in these cells in a dose- and time-dependent manner compared to control cells, and fucoidan downregulated TGFR expression over One mechanism by which fucoidan-induced reduction of TGFRI and TGFRII proteins in NSCLC may occur is through degradation via the ubiquitin-dependent proteasome pathway (UPP). In cells cultured with fucoidan (200 μg/ml), TGFRI and TGFRII protein levels were reduced by 30% and 50%, respectively. They also found that adding a proteasome inhibitor, MG-132, to cells could prevent fucoidan-induced TGFRI and TGFRII proteolysis compared to cells treated with fucoidan alone.
These results suggest that the proteasome-dependent pathway is involved in fucoidan-induced TGFRI/II proteolysis. The study included carrying out an in vitro ubiquitination activity assay on ubiquitin protein to further investigate how fucoidan-induced TGFRI/II protein reduction occurs. Our goal was to determine the involvement and significance of ubiquitin (ubiquitination) in the proteasomal degradation of TGFR protein in NSCLC (CL1-5 and A549). Cells were preincubated with MG-132 and treated with fucoidan, and then the cell lysates were incubated with an anti-TGFR antibody. The immunoprecipitated proteins were then evaluated using an anti-ubiquitin antibody. The findings indicate that denatured TGFRI/II protein can be identified using an anti-ubiquitin antibody, suggesting that ubiquitination of TGFRI/II protein occurs during the reduction of TGFR protein induced by fucoidan. Overall, the results strongly indicate that the smear bands observed are a result of polyubiquitinated TGFRI and II proteins. This suggests that the ubiquitin-proteasome pathway (UPP) is involved in the degradation of TGFR, which is enhanced by fucoidan, in NSCLC within a 24-hour timeframe.
The findings of this study identify for the first time a novel mechanism of the antitumor activity of fucoidan, namely, suppressing tumor growth by regulating the TGFR/Smad7/Smurf2-dependent axis, leading to the degradation of TGFR protein and inhibition of lung cancer cell progression in vitro and in vivo. This suggests that fucoidan works by using Smurf2-dependent ubiquitin degradation of TGFβ receptors, making it a possible treatment option or dietary supplement for lung cancer.
Source: Oncotarget 2014 Aug 6;5(17):7870–7885. doi: 10.18632/oncotarget.2317
