The active crossing of the blood-brain barrier can be induced by P-selectin-targeted nanocarriers through caveolin-1-dependent transcytosis, which serves as a mediator in drug delivery through the blood-brain barrier. It is worth noting that Medulloblastoma, the most common malignant childhood brain tumor, is influenced by Sonic Hedgehog signaling in approximately 30% of cases. Vismodegib, immune therapy-mediated inhibition of the Sonic hedgehog effector Smoothened, inhibits tumor growth, but at effective doses causes growth plate fusion, stops the growth of a bone.
It is worth mentioning that fucoidan-based nanoparticles have been proven to be non-toxic, making them a safe option for medical applications. Moreover, these nanoparticles have the remarkable ability to easily cross the blood-brain barrier, a discovery that has been validated by experts from both the Mount Sinai Health System and Memorial Sloan Kettering Cancer Center. (https://www.genengnews.com/news/nanoparticles-target-medulloblastoma-tumor-to-improve-drug-therapy-and-reduce-toxicity/)
Hence, in this blog, I would like to share an extensive study, “P-selectin-targeted nanocarriers induce active crossing of the blood-brain barrier via caveolin-1-dependent transcytosis” by Daniel E. Tylawsky et al. The study discusses a nanotherapeutic approach that is based on fucoidan and targets the vasculature of endothelial tumors in order to improve the ability to cross the blood-brain barrier.
First, they characterized the cerebral vasculature in a genetically engineered mouse (GEM) model to investigate BBB integrity. Symptomatic mice (14 weeks of age or older) were injected intravenously with tetramethylrhodamine (TMR) dextran. The researchers noted that there was minimal extravasation of TMR-dextran into the parenchymal brain tumor tissue, and this observation remained consistent even after administering low-dose ionizing radiation. Based on their findings, they come to the conclusion that the BBB of these mice remains intact even during advanced tumor stages, making it comparable to the physiological condition observed in human patients with SHH-MB6.
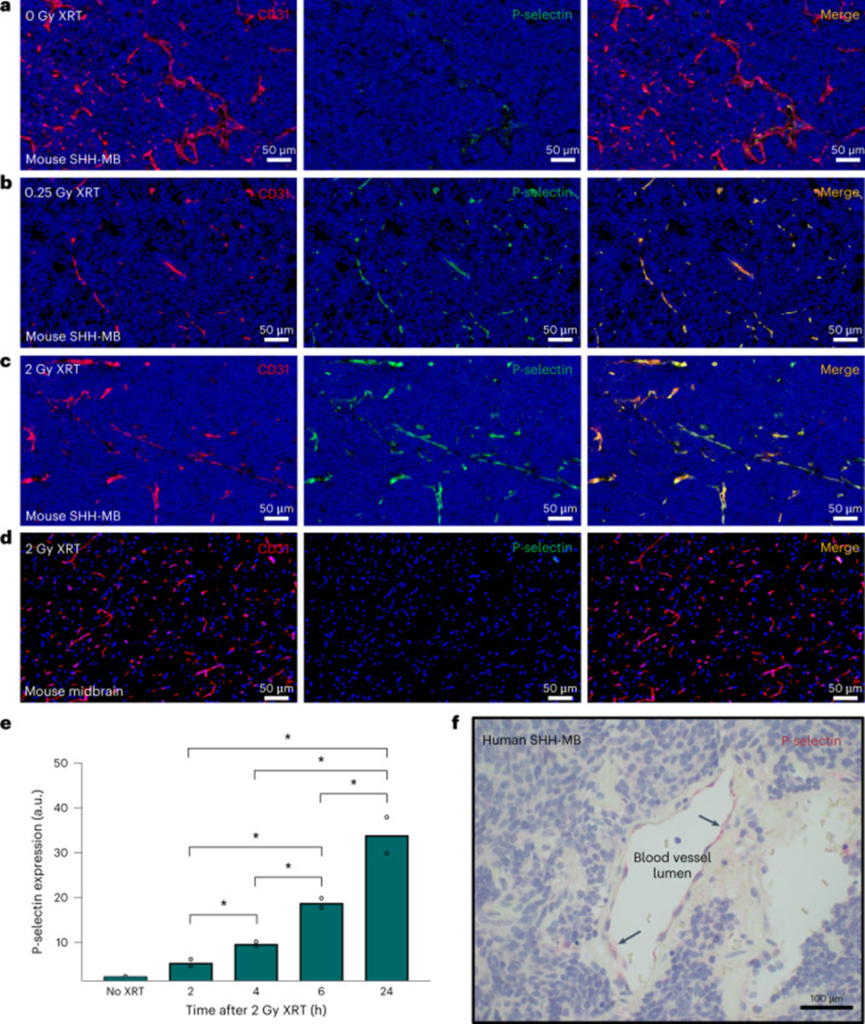
Their investigation progressed to analyzing the expression of her P-selectin within the tumor microenvironment of SHH-MB, followed by an examination of the potential impacts of low-dose X-ray irradiation (XRT). In the absence of radiation, the study demonstrates the expression of P-selectin in the tumor vasculature of SHH-MB, as evidenced by Figure 1a. Moreover, after a single XRT irradiation of 2 Gy, the research findings indicate a significant increase in P-selectin expression, as illustrated in Figure 1c. The rise in P-selectin expression post whole-brain irradiation was limited to the area affected by the tumor, with no discernible impact on the surrounding healthy brain tissue.
To evaluate the possibility of mitigating RT-related toxicity, we found that P-selectin expression could still be strongly induced after a single irradiation of 0.25 Gy XRT (Fig. 1b). P-selectin expression was observed to reach substantially elevated levels approximately 6 h after XRT, and these levels persisted for at least 24 h (Fig. 1e). To confirm the clinical relevance of endothelial P-selectin expression as a potential target molecule, we examined human SHH-MB tumor tissue surgically excised from pediatric patients. The vasculature neighboring the tumor was found to express P-selectin, as evidenced by the immunohistochemical analysis shown in Figure 1f.
In order to direct SHH pathway regulators to P-selectin, nanoparticles were developed that enclose the Smoothened inhibitor vismodegib. They used a nanoprecipitation process incorporating the polysaccharide fucoidan to target P-selectin and a near-infrared dye (IRDye783) to facilitate imaging. The FiVis nanoparticles that were obtained had an average size of 80 ± 10 nm, which was determined through various techniques such as atomic force microscopy, dynamic light scattering (DLS), and nanoparticle tracking analysis. These characterization methods confirmed that the nanoparticles were relatively homogeneous in nature. FiVis nanoparticles were also tested for stability in serum to evaluate nanoparticle localization and RT effects on SHH-MB tumors.
The use of FiVis nanoparticles in non-irradiated mice resulted in a slight increase in targeting to cerebellar tumors, as opposed to adjacent normal forebrain tissue. In mice that received radiation pretreatment (1 Gy), we observed a significant buildup of tumors, with FiVis luminescence specifically confined to the blood vessels, which was not the case in the tumor-free WT mice. Furthermore, the researchers synthesized nanoparticles called DexVis, which were based on control dextran sulfate, and interestingly, these particles did not exhibit any affinity towards P-selectin.
Despite having similar size and drug encapsulation properties as FiVis, these nanoparticles did not show the same ability to localize in tumors, even when exposed to irradiation. By altering the radiation dose, researchers noted a substantial increase in both P-selectin enhancement and tumor accumulation of FiVis NPs. The effectiveness of treatment with 0.25 Gy XRT in terms of localizing his FiVis nanoparticles at the tumor site was remarkable, exhibiting a more than three-fold increase compared to the adjacent normal forebrain tissue. It was noted that the signal emitted by the nanoparticles exhibited a positive correlation with the dosage administered, further emphasizing the potential of this approach.
The presence of P-selectin is crucial for the penetration of FiVis nanoparticles into tumors, as evidenced by the lack of localization in P-selectin knockout (KO) SHH-MB mice. To assess the fate of nanoparticle drug cargo, vismodegib was measured by liquid chromatography-mass spectrometry (LC-MS). In mice treated with FiVis and 0.25 Gy XRT, we found a substantial preferential accumulation of drug within the cerebellar tumor region compared to the adjacent normal forebrain region.
These findings have the potential to overcome the challenging blood-brain barrier, allowing for better penetration into tumors. This can increase the effectiveness of treatment while reducing the risk of neurotoxicity and overall toxicity. It also opens up possibilities for treating diseases within the central nervous system, presenting a promising approach for targeted drug delivery to the brain.
Source: Nat Mater. 2023; 22(3): 391–399. doi: 10.1038/s41563-023-01481-9
