Diabetic nephropathy, one of the complications of diabetes, is a disease in which hyperglycemia destroys the kidneys and reduces renal function, ultimately leading to end-stage renal failure requiring dialysis or kidney transplantation.
A previous study proved that Fucoidan (FPS) has biological activity and potential applications, including anti-inflammatory and anti-cancer effects, immune response, and pathogen inhibition. Additionally, FPS is widely used to treat renal fibrosis (RF) in patients with diabetic kidney disease (DKD). However, the exact therapeutic mechanism remains unclear.
So, I would like to share the following study with you: “Fucoidan Alleviates Renal Fibrosis in Diabetic Kidney Disease via Inhibition of NLRP3 Inflammasome-Mediated Podocyte Pyroptosis” by Mei-Zi Wang et al. In this study, we used a modified DKD rat model and murine podocytes to assess the ameliorative effects of FPS on RF and inflammatory podocyte injury in vivo and in vitro and to clarify the anti-RF mechanisms of FPS via targeting NLRP3 inflammasome-mediated podocyte pyroptosis in the diabetic kidney. They uncovered an anti-RF mechanism of FPS by targeting cell pyroptosis.
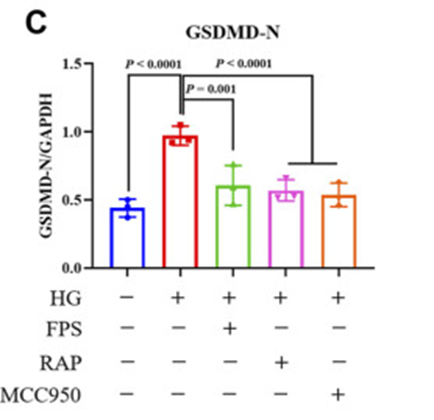
Firstly, a modified DKD rat model was subjected to a single nephrectomy, intraperitoneal injection of streptozotocin, and a high-fat diet. After induction of renal injury, animals received FPS, rapamycin (RAP), or vehicle for four weeks. Hence, they exposed mouse podocytes to high glucose (HG) and MCC950, NLRP3 inflammasome inhibitor, with or without FPS or RAP. Then FPS or RAP treatment significantly ameliorated these changes in HG-exposed podocytes compared to HG treatment alone. Furthermore, they also discovered improved protein expression levels of GSDMD-N in podocytes exposed to HG and MCC950 compared to podocytes treated with HG alone. (See Figure 1C)
Changes in parameters associated with RF and inflammatory podocyte injury were also analyzed in vivo. Involved in these changes in podocyte pyroptosis, activation of the NLRP3 inflammasome and activation of the adenosine monophosphate-activated protein kinase (AMPK)/mammalian target of rapamycin complex 1 (mTORC1)/NLRP3 signaling axis changes were analyzed in vivo and in vitro. As a result, FPS and RAP ameliorated RF and inflammatory podocyte damage in DKD model rats. Furthermore, FPS and RAP attenuated podocyte pyroptosis, inhibited NLRP3 inflammasome activation, and modulated the AMPK/mTORC1/NLRP3 signaling axis in vivo and in vitro.
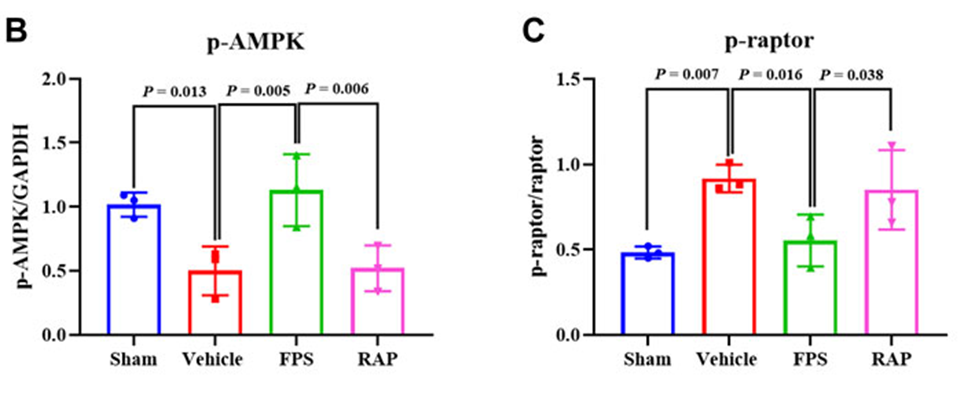
In particular, the data suggest that crucial signaling molecules such as p-AMPK and p-raptor in the AMPK/mTORC1/NLRP3 signaling axis, both in vivo and in vitro, are closely related to the regulatory effects of FPS. They have shown the regulatory outcomes of FPS to be better than the regulatory effects of RAP. (See Figure. 2 B. C)
Thus it confirms that FPS, like RAP, can attenuate RF in DKD by inhibiting NLRP3 inflammasome-mediated podocyte pyroptosis through modulation of the AMPK/mTORC1/NLRP3 signaling axis in the diabetic kidney. The findings provide a detailed understanding of RF pathogenesis, and they found FPS was useful for DKD therapy.
Source: Int J Mol Sci. 2018 Dec; 19(12): 4050. doi: 10.3390/ijms19124050