Fucoidan, a type of polysaccharide found in brown algae, has gained significant interest due to its anti-inflammatory properties.
The purpose of this blog post is to provide you with information about a study titled “Formulation, Optimization and In Vivo Evaluation of Fucoidan-Based Cream with Anti-Inflammatory Properties” conducted by Ekaterina D. Obluchinskaya et al. This study focuses on the development of a cream formulation that utilizes fucoidan extracted from the brown alga Fucus vesiculosus.
In order to achieve its goals, the current study focused on two main aspects. Firstly, the development and optimization of a cream formulation that utilized fucoidan as its primary component. Secondly, the investigation of the cream’s potential to reduce inflammation when applied topically on the skin.
Fucoidan does not form a stable gel when mixed with water. Thus, it becomes necessary to include other polymers in order to achieve the desired outcome. Olive oil was chosen as the oil phase of the cream base. Olive oil easily penetrates the epidermis of the skin, allowing good absorption of pharmaceuticals. Corifol RH40 was added to stabilize the water-in-oil (o/w) emulsion.
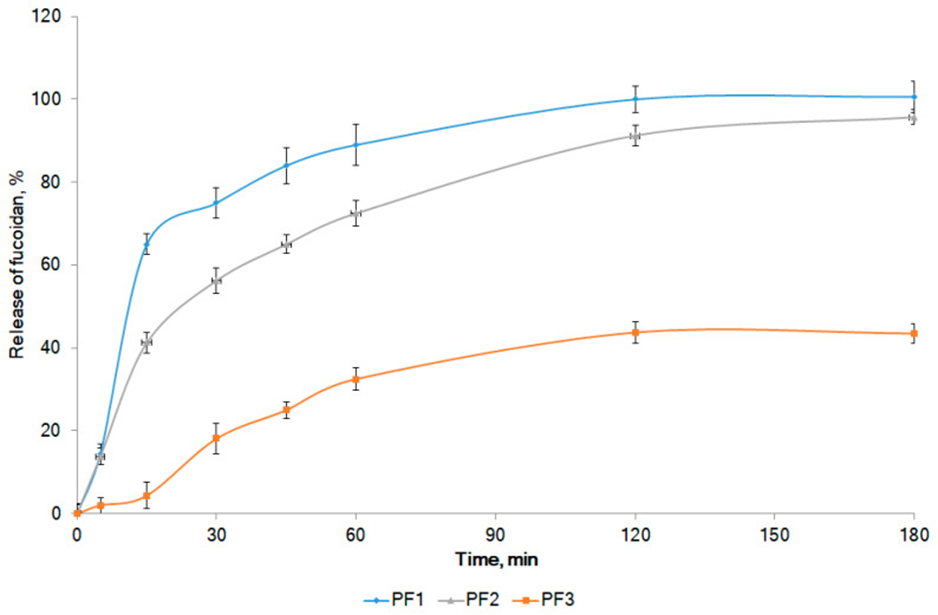
The research involved analyzing the release of fucoidan from the model formulations and assessing the quality of the formulations through the study of its diffusion into agar gel. Diffusion from model formulations PF1 and PF2 began within the first hour, and after 18 hours the diffusion area expanded to 2.5 ± 0.1 mm. The in vitro release profiles of fucoidan from the model compositions are shown in Figure 1.
The formulation containing dimethyl sulfoxide (PF1D1) showed a release rate of approximately 65% of fucoidan within the first hour. Interestingly, there was no additional release observed during the subsequent 2 hours, which suggests that the bioavailability of fucoidan from PF1D1 is relatively low. The active ingredient was released well when polysorbate 80 (PF1P1) and transcutol P (PF1T1) were used as penetrants. After 1 hour, approximately 90% of fucoidan was released from both formulations.
PF1T1P5 and PF1P1T5 formulations were prepared by adding 5% PEG400 to PF1T1 and PF1P1, respectively. PF1T1G5 and PF1P1G5 formulations were prepared by adding 5% glycerol to PF1T1 and PF1P1, respectively. All formulations were colloidally stable and did not irritate the skin. The PF1T1P5 formulation showed great potential due to its wide coverage and spreadability, creating a uniform brown cream with a distinct smell.
Data from the stability study of the fucoidan-based cream showed that the concentration of fucoidan, its release, and the colloidal stability of the cream did not change after 365 days of storage at room temperature or cold temperature (5 ± 3 °C) (p > 0.05, n = 6), demonstrating the high physical stability of the formulation.
The anti-inflammatory effects of the new fucoidan cream were tested using two animal models: rats with paw swelling induced by carrageenan and a test for increased sensitivity to touch. Following the topical application of the fucoidan-based cream, there was a clear dose-dependent inhibition of paw edema, as shown in Figure 4. A statistically significant inhibition of paw edema was observed in both diclofenac gel and fucoidan cream groups (400 mg/rat) from the third day of the experiment (analysis of variance (ANOVA) F test, p < 0.05). Interestingly, on the fifth day, the inhibition of edema by the fucoidan-based cream (400 mg/rat) exceeded 50%. It is noteworthy that the efficacy of the high dose of the fucoidan-based cream was comparable to that of the diclofenac gel.
The effectiveness of the newly created fucoidan cream in reducing inflammation was tested using two animal models: one inducing rat paw swelling with carrageenan and another assessing sensitivity to touch. The fucoidan-based cream dose-dependently inhibited paw edema after topical application. A statistically significant inhibition of paw edema was observed in both diclofenac gel and fucoidan cream groups (400 mg/rat) from the third day of the experiment. Interestingly, on the fifth day, the inhibition of edema by the fucoidan-based cream (400 mg/rat) exceeded 50%. It is worth mentioning that the high dose of the cream containing fucoidan showed similar effectiveness to the diclofenac gel.
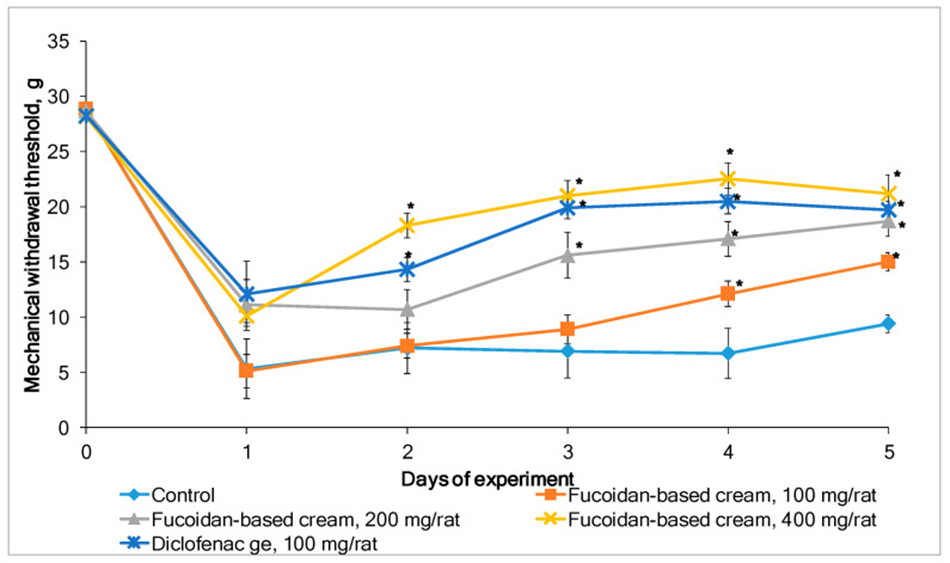
The mechanical withdrawal response of inflamed rat paws in the von Frey hair model was used to examine whether the fucoidan-based cream reduces mechanical allodynia. Figure 2 shows the inflammatory pain response (mechanical withdrawal threshold) in rats after topical application of placebo (control group), fucoidan-based cream, or diclofenac gel. Repeated application of the fucoidan-based cream for 5 consecutive days dose-dependently alleviated carrageenan-induced allodynia in rats. There were no signs of irritation or any other adverse effects observed when using the fucoidan-based cream for the 5-day treatment in both rat inflammation models.
The aim of this study was to examine the potential anti-inflammatory effects of a cream containing fucoidan derived from Fucus vesiculosus in rat models of carrageenan-induced rat paw edema and rat mechanical allodynia. This is the first study to investigate this particular aspect. By examining the findings in conjunction with literature data, it can be concluded that fucoidan is a preferred compound for topical use over injection because of its convenience, strong patient compliance, and localized effects. Given its slow onset of effect, they believe that topical application of a fucoidan-based cream may be beneficial for the treatment of chronic inflammatory diseases. Additional studies are required to validate this.
Source: Mar Drugs. 2021 Nov; 19(11): 643. doi: 10.3390/md19110643
