Pancreatic cancer, specifically pancreatic adenocarcinoma, which is the most common type, is known for its dismal prognosis, making it the fourth leading cause of cancer-related death. Hypoxia serves as a prominent mechanism of treatment resistance in pancreatic cancer. Therefore, it is imperative to develop innovative therapeutic interventions that can promptly tackle and mitigate the effects of hypoxia.
Tumor hypoxia is widely acknowledged as a prominent mechanism of resistance to radiation therapy (RT) and is closely associated with a poor prognosis in cancer patients affected by this condition. The treatment of hypoxic and radiation-resistant cancers, including pancreatic cancer, has been met with limited success using different approaches. On the other hand, Fucoidan, which is a polysaccharide derived from brown seaweed, exhibits remarkable antitumor and antiangiogenesis properties.
Hence in this blog, I would like to introduce the research, “Fucoidan-Manganese Dioxide Nanoparticles Potentiate Radiation Therapy by Co-Targeting Tumor Hypoxia and Angiogenesis” by Sung-Won Shin et al. Given this situation, there is an urgent need for the development of new therapeutic strategies to combat hypoxia. Nanomedicine’s application to cancer therapy is gaining increasing attention, mainly because it offers several advantages, such as enhanced drug delivery to tumors.
In the study, there is a description of the development of fucoidan-coated manganese dioxide nanoparticles (Fuco-MnO2-NPs) and the testing of their therapeutic potential by RT using a pancreatic cancer model.
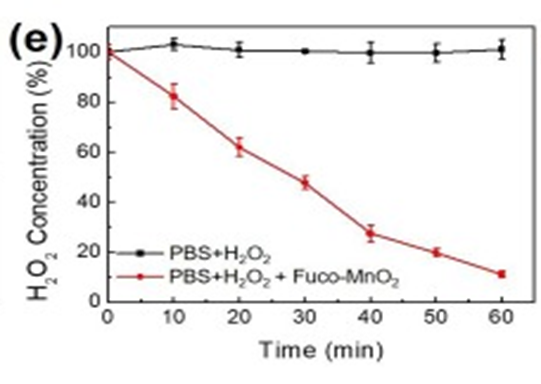
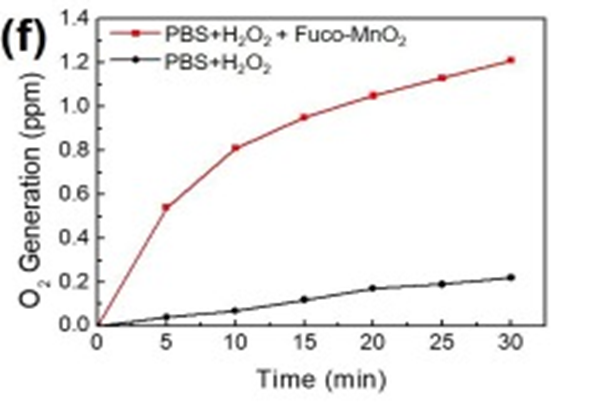
First, after fucoidan coating, the reactivity of Fuco-MnO2-NPs was evaluated using a time-dependent H2O2 assay after the addition of NPs. Within 60 min, 2.5 mM H2O2 was almost completely degraded by 250 μM Fuco-MnO2-NPs. (See Figure. Following that, they examined O2 generation at reduced concentrations of H2O2 (250 μM) in hypoxic conditions. The presence of a considerable amount of oxygen was observed as a result of the heightened activity of Fuco-MnO2-NPs. (See Figure. 1f) The obtained results demonstrate that the introduction of fucoidan coating does not alter the reactivity of MnO2-NPs towards H2O2.
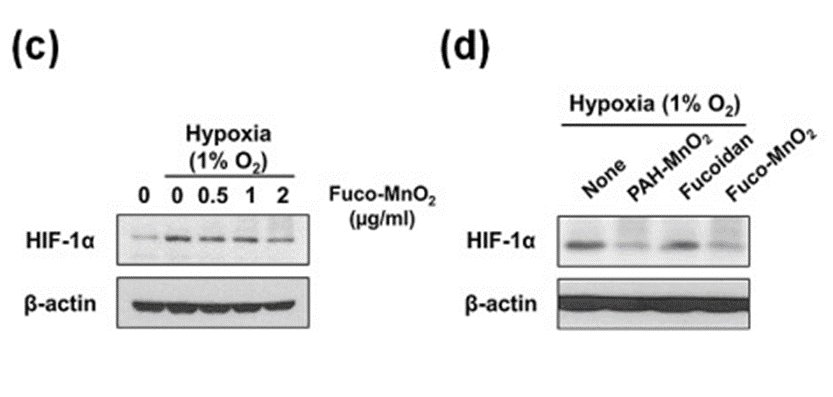
Next, to measure the radiosensitizing effect of Fuco-MnO2-NPs on hypoxic tumor cells, fucoidan alone was tested by measuring the increase in luminescence using a hypoxia-responsive luciferase activity assay. Treatment with PAH-MnO2-NPs suppressed hypoxia-induced HIF-1α expression (See Figure. 2c, d) or hypoxia-responsive luciferase activity in pancreatic cancer cells. Fuco-MnO2-NPs were found to alleviate hypoxia via the production of oxygen. The data obtained from in vitro experiments demonstrated that Fuco-MnO2-NPs have the ability to effectively produce oxygen when exposed to His H2O2, and they also exhibit a significant inhibitory effect on the expression of Her HIF-1 under hypoxic conditions in human pancreatic cancer cells.
The elucidation of the underlying mechanism of radiosensitization by Fuco-MnO2-NPs involved studying the repair process after irradiation, where it was found that some lesions were damaged as a result. The findings indicate that the count of γ-H2AX foci at 24 hours after irradiation was lower in hypoxic conditions compared to normoxic conditions, highlighting the role of oxygen in the repair of DNA damage. PAH-MnO2-NPs or Fuco-MnO2-NPs increased the number of γ-H2AX foci 24 h after irradiation compared with saline controls or fucoidan. Thus, based on the data, it can be inferred that Fuco-MnO2-NPs have the potential to inhibit DNA damage repair by enhancing the oxygen effect, resulting in the radiosensitization of BxPC-3 cells.
Fuco-MnO2-NPs reversed hypoxia-induced radio-tolerance by reducing clonogenic viability and increasing DNA damage and apoptotic cell death in response to RT. The BxPC3 xenograft mouse model demonstrated that the combined treatment of Fuco-MnO2-NP and RT resulted in a more significant delay in tumor growth than when RT was used alone. According to the immunohistochemical data, the Fucoidan-coated nanoparticles (NPs) exhibited a greater ability to suppress tumor angiogenesis compared to the bare NPs. This was evidenced by the decreased expression of phosphorylated vascular endothelial growth factor receptor 2 (VEGFR2) and CD31. The data strongly suggests that Fuco-MnO2-NPs can greatly enhance the efficacy of radiotherapy in the treatment of hypoxic, radioresistant pancreatic cancer. This is achieved through their dual targeting of tumor hypoxia and angiogenesis, indicating their clinical relevance and potential.
2.5 mM H2O2 was almost completely degraded by 250 μM Fuco-MnO2-NPs.
Source: Mar Drugs. 2018 Dec; 16(12): 510. doi: 10.3390/md16120510
