The pathogenesis of ALD (Alcohol Liver Disease) is known to be complicated. Additionally, the occurrence of lipid metabolism disorders, oxidative stress, and inflammatory reactions have been confirmed in the studies. Alcohol-induced cytochrome P450-2E1 (CYP2E) can oxidize excess amounts of ethanol to acetaldehyde and generate large amounts of reactive oxygen species (ROS), leading to mitochondrial damage and accelerated fatty liver formation. ROS also leads to the activation of Kupffer cells (KCs), which is activated KCs trigger an inflammatory cascade, releasing interleukin-1β (IL-1β), IL-6, IL-18, and other pro-inflammatory cytokines to exacerbate liver injury.
Fatty liver is an early stage of ALD and is a reversible condition. Long-term ethanol exposure can cause irreversible diseases such as steatohepatitis, fibrosis, cirrhosis, and even hepatocellular carcinoma. Unfortunately, there is currently no specific medicine.
On the other hand, fucoidan has the beneficial outcome of anti-inflammatory, antioxidative, and antitumor. Hence, in this blog, I would like to inform you that the study focuses on fucoidan, “Effect of fucoidan on ethanol-induced liver injury and steatosis in mice and the underlying mechanism,” by Meilan Xue et al., examined to use of an alcoholic liver injury mouse model. They investigated the effect of fucoidan on ethanol-induced liver injury and steatosis and its underlying mechanisms.
First, all mice were randomly divided into four groups: 1) Control group, 2) Model group, 3) Diammonium glycyrrhizinate treatment group (200 mg/kg body weight), and 4) Fucoidan that was extracted from Fucus vesiculosus treatment group (300 mg/kg body weight) and fed eight-week administration of ethanol-induced liver injury and steatosis in mice. The results showed that dietary fucoidan had hepatoprotective effects in ethanol-fed male C57BL/6J mice for eight weeks. Fucoidan treatment decreased serum ALT, CHOL, and liver TG levels. (See Figure.1) Furthermore, the morphology of hepatocytes was improved after fucoidan treatment.
p-AMPKα, SIRT1, ChREBP, PGC-1α, and HNF-1α regulate hepatic fat metabolism and inflammatory responses. Hence, they were analyzed for liver expression to understand the mechanisms underlying the protective effects of fucoidan against ethanol-induced liver injury and steatosis. Western blot results showed that ethanol intake suppressed p-AMPKα, SIRT1, and PGC-1α, thereby promoting alcoholic fatty liver. However, fucoidan treatment reversed the effect of ethanol on the expression of p-AMPKα, SIRT1, and PGC-1α. (See Figure 2)
Similarly, ethanol-fed mice showed significantly higher hepatic ChREBP and HNF-1α levels than mice in the control group. In contrast, mice in the fucoidan group showed significantly reduced levels of ChREBP and HNF-1α (P < 0.05) than mice in the model group. Fucoidan treatment suppressed the expression of ChREBP and HNF-1α by activating the AMPK-α1/SIRT1 pathway, upregulated PGC-1α, exerted anti-inflammatory and antioxidant effects, and decreased lipid accumulation. Thus, alleviating chronic alcoholic liver injury.
Also, chronic alcohol administration increased hepatic and serum bile acid levels and synergized with alcohol to increase the severity of liver damage, and was found to increase bile acid levels. However, treatment with fucoidan resulted in decreased hepatic TBA ( Serum total bile acid) levels. These results suggest that fucoidan may ameliorate alcohol-induced disorders of bile acid metabolism.
The study also revealed that fucoidan inhibits alcohol-induced steatosis and impaired bile acid metabolism via the AMPK/SIRT1 pathway and the gut microbiota-bile acid-liver axis. This data suggests that fucoidan may effectively prevent and treat ALD.
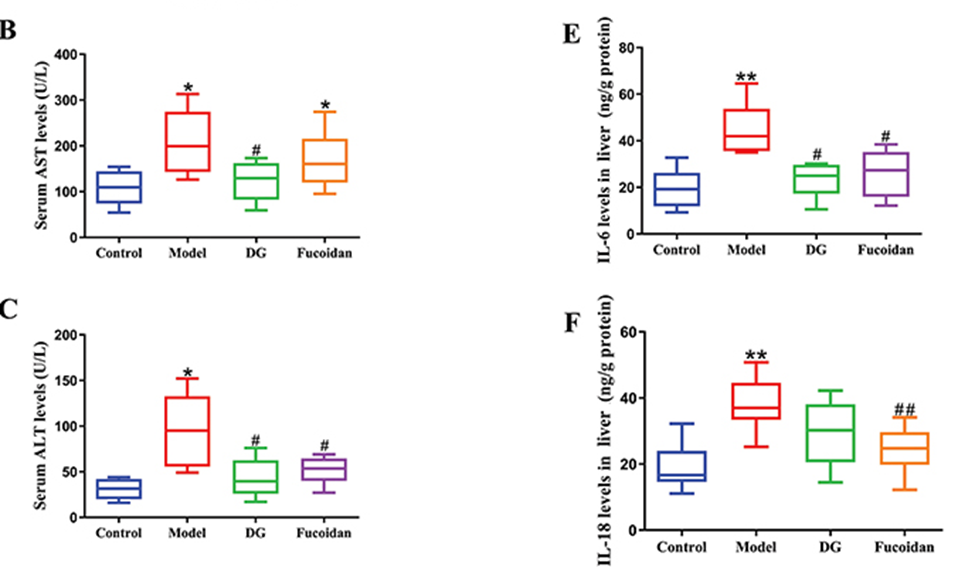
Figure. 1) Fucoidan treatment decreased serum ALT, AST, and cytokine levels
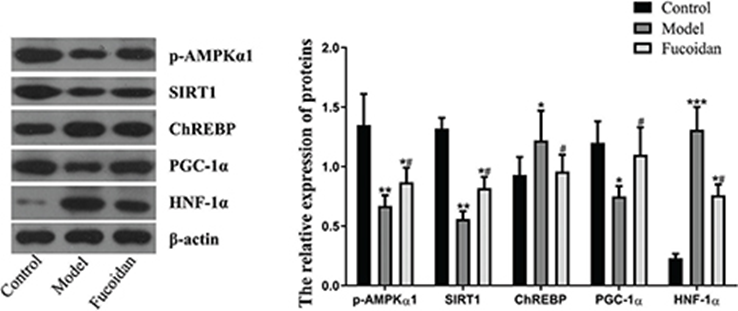
Figure. 2) Effect of fucoidan on the AMPK/SIRT1 signaling pathway
Source: 2021; 65: 10.29219/fnr.v65.5384. doi: 10.29219/fnr.v65.5384
