In spite of promising results, traditional anticancer therapies decrease the patient’s Quality of life (QOL) with selective eradication and associated side effects. Hence, I would like to share the fucoidan’s effect that positively increases cancer patients’ Quality of life through the following study, “An Exploratory Study on the Anti-inflammatory Effects of Fucoidan in Relation to Quality of Life in Advanced Cancer Patient” by Hidenori Takahashi, MD et al.
Fucoidan is extracted from seaweed and is known to have a wide range of biological activities, such as anti-inflammatory, anti-virus, and anticancer effects. However, there have been few clinical trials using fucoidan despite its potential benefits. Thus, they conducted an exploratory clinical trial in patients with advanced cancer to investigate the efficacy of fucoidan. It mainly focuses specifically on inflammation associated with Quality of life scores. Patient characteristics vary greatly depending on the primary origin of cancer. However, they all have distant metastases, and 90% of patients already had standard chemotherapy for advanced-stage cancer before fucoidan intake.
So they conducted a prospective open-label clinical trial and recruited 20 patients with advanced cancer with metastasis. Then gave them fucoidan extract from the brown seaweed Mozuku, Cladosiphon novae-caledonia Kylin, used in the present study by oral administration. The patients were given 400 mL / d fucoidan (10 mg / mL) for at least four weeks. Inflammatory biomarkers, including sensitive C-reactive proteins and various cytokines, and Quality of life scores were monitored before testing, after two weeks, and after four weeks of fucoidan intake.
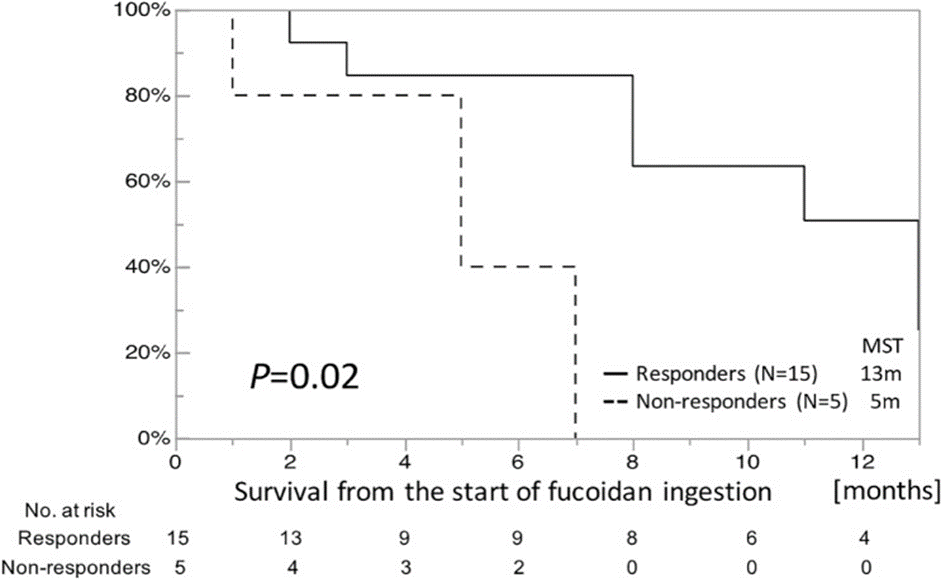
As a result, blood cell counts, including white blood cells and sensitive CRP, were stable during the study. Also, three major inflammatory cytokines, IL-1β, IL-6, and TNF-α, were significantly reduced after two days. Univariate analysis by log-rank test showed that patients with decreased IL-1β levels in the first two weeks (IL-1β responders) had a significantly longer survival rate (survival time) compared to the prognosis of IL-1β non-responders was shown.
Responsiveness of other inflammatory biomarkers, including sensitive CRP, IL-6, and TNF-α, compared to IL-1β, did not correlate with survival. Multivariate analysis using the Cox proportional hazards regression model also revealed that IL-1β responsiveness was the only significant independent prognostic factor in all 20 patients (See Figure 1).
In this experimental prospective clinical trial in patients with advanced cancer, it became clear that critical inflammatory cytokines such as IL-1β, IL-6, and TNF-α were significantly reduced after short-term fucoidan administration.
Interestingly, subgroup analysis showed that IL-1β reactivity was significantly correlated with overall survival, a helpful prognostic biomarker for patients with advanced cancer receiving fucoidan. They suggested that it could be, as far as knowing, it is the first study to provide evidence of the anti-inflammatory effect of fucoidan on patients with advanced cancer.
Finally, IL-1β, IL-6, and TNF-α levels decreased two weeks after fucoidan administration but returned to pretreatment levels four weeks later. Possibly the dose of fucoidan was inadequate, the treatment was too short, or the 4-week monitoring time was just the transition from cytokine markers to other markers.
Based on my experience as a naturopathy doctor, I think it involved a dose-dependent matter.
In the future, more extensive controlled trials are needed to establish the efficacy of fucoidan, especially in patients with advanced cancer who are receiving Cx as supportive care.