The United States has been seeing growing issues with illegal immigrants coming from nearby countries. They are mainly coming from Latin America. These nations are known for various tuberculosis cases, especially high rates of Tuberculosis in large cities and prisons, with half of all illnesses in Peru, Brazil, and Mexico. Therefore, we have to prepare new strategies, a new approach based on “Tuberculosis Genetic Epidemiology a Latin American Perspective” by Marc Woodman et al.
Tuberculosis is an infectious disease caused by infection with Mycobacterium tuberculosis. Inhaling tubercle bacilli present in the air triggers an infection (airborne infection), which primarily affects the lungs. Mycobacterium tuberculosis taken up by the lungs may linger in the lungs without causing any symptoms. However, when the immune system of an infected person is weakened, it starts to work and causes symptoms.
In addition, tubercle bacilli may spread throughout the body and the flow of blood and lymph, causing more severe miliary Tuberculosis and tuberculous meningitis. With modern medication, antibiotics and other drugs are given as therapeutic agents, whereas some surviving tubercle bacilli develop resistance. So, the drug does not work for tubercle bacilli with acquired resistance. These medicines also cause side effects such as liver damage. For these reasons, it is crucial to improve our own immunity.
On the other hand, fucoidan, a polysaccharide extracted from brown algae, has several physiological benefits, such as immunomodulation, anti-inflammation, anti-viral, and anti-tumor, without any side effects.
In this blog, I would like to discuss the fucoidan effect on Tuberculosis using the study “ Inhalable Fucoidan Microparticles Combining Two Antitubercular Drugs with Potential in Pulmonary Tuberculosis Therapy” by Ludmylla Cunha et al.
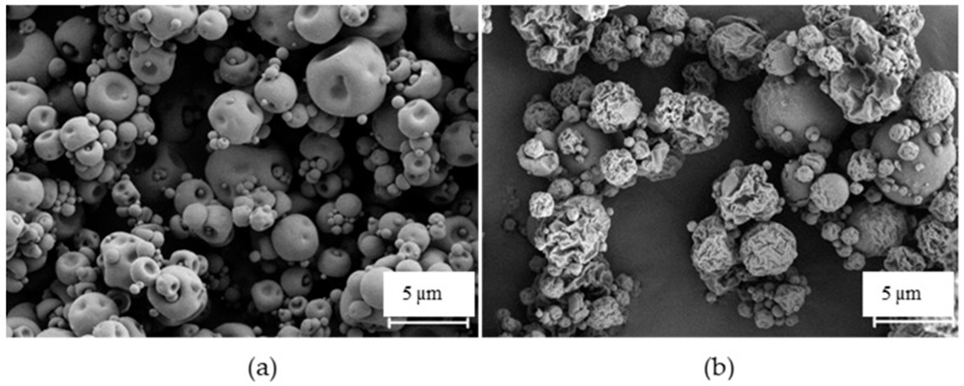
Fucoidan is a polysaccharide composed of chemical units that have been reported and recognized explicitly by alveolar macrophages (hosts of mycobacteria). They successfully produced inhalable fucoidan particles and effectively bound isoniazid (97%) and rifabutin (95%) simultaneously. Additionally, the particles exhibited aerodynamic properties suitable for pulmonary delivery that could reach the respiratory zone, with a median aerodynamic diameter (MMAD) of 3.6-3.9 µm.
The produced microparticles were further captured by macrophage-like cells (23%–87% uptake) in a dose-dependent manner. Moreover, they can induce macrophage activation by potentiating the secretion of cytokines. Similarly, drug-loaded microparticles showed potential activity against a strain of mycobacteria (95% growth inhibition). (See Fig. 1)
The drug did not demonstrate a cytotoxic effect on lung epithelial cells (A549), but mild toxicity was observed on macrophage-differentiated THP-1 cells at the highest tested concentrations.
Fucoidan microparticles also showed a dose-dependent tendency to be captured by macrophages and their ability to activate target cells. In addition, loaded with drugs, the microparticles effectively inhibited the growth of mycobacteria in vitro. Thus, the fucoidan particles produced are thought to have potential as a pulmonary delivery system for treatment.
According to the obtained data, the proposed delivery carrier, combining two antitubercular drugs in a single formulation, is a promising option for the inhalable treatment of pulmonary TB.